February 27, 2018 -- San Diego, CA -- Okogen, Inc., a developer of ophthalmic anti-infective drugs, announced $10 million in Series A funding from Brandon Capital’s Medical Research Commercialisation Fund (MRCF) to advance the ongoing development of the company’s lead candidate for viral conjunctivitis (commonly known as pink eye), OKG-0301. There are approximately 30 million cases of acute conjunctivitis annually worldwide, including 6 million cases in the United States alone. Seventy percent of U.S. conjunctivitis patients seek treatment each year, of which 80% have viral forms of the infection.
“There are no approved therapies for viral conjunctivitis, and patients frequently receive antibiotics despite their lack of efficacy in treating viral infections and potential to cause antibiotic resistance,” said Brian M. Strem, Chief Executive Officer of Okogen. “Safe and effective treatment options are needed to address this highly contagious and common infection, and we believe that OKG-0301 has substantial clinical and commercial potential in this indication. This Series A funding will support our OKG-0301 clinical development plan, including initiation of a Phase 2 clinical trial before the end of 2018. We believe that the support of Brandon Capital, a fund with deep expertise in identifying promising biopharmaceutical companies, highlights Okogen’s exciting opportunities.”
OKG-0301 is an ophthalmic formulation of ranpirnase, a potent ribonuclease with established broad-spectrum antiviral properties. Adenoviral conjunctivitis, the initial clinical indication in which OKG-0301 will be evaluated, is the number one cause of eye infections globally. The infection causes significant patient morbidity and leads to millions of visits to emergency departments, urgent care facilities, ophthalmic clinics and pediatric departments in the United States alone. With no approved therapies, adenoviral conjunctivitis has significant unmet medical need throughout the world.
Okogen Board Director and MRCF Investment Manager Chris Smith said, “Our investment in Okogen demonstrates our belief in the company’s technology, team and potential to positively impact the lives of millions of patients.”
In addition to the Series A Funding, Okogen also announced the appointment of Quinton Oswald to the Board of Directors. Mr. Oswald currently serves as the President and CEO of Notal Vision, a commercial-stage ophthalmic company focused on age-related macular degeneration. Prior to Notal, Mr. Oswald was President and CEO at Neurotech Pharmaceuticals and SARcode Bioscience. Mr. Oswald has also held executive level roles at Genentech, Novartis and Bristol-Myers Squibb. Added Mr. Strem, “Quinton’s history of leadership and accomplishment in the ocular space is unparalleled, and we are excited to benefit from the knowledge and experience he brings to Okogen.”
About OKG-0301
OKG-0301 is based on ranpirnase, the active pharmaceutical ingredient (API) previously advanced to late stage clinical trials in oncology. As an IV formulation, this API has been administered to over 800 patients as part of clinical testing, in addition to a significant amount of preclinical toxicology and CMC. These data highlight a strong safety profile, as well as a validated, commercial-scale manufacturing process that will accelerate OKG-0301’s path to the clinic.
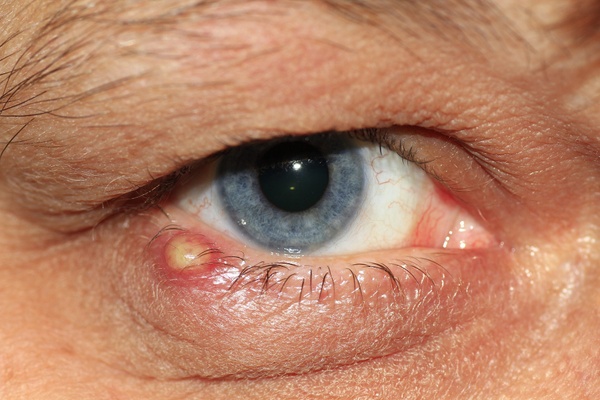
About Adenoviral Conjunctivitis
Worldwide, adenoviral conjunctivitis is the most common eye infection, affecting approximately 30 million individuals annually. The condition is a leading ocular disease, responsible for over 1% of all primary care visits. Most cases involve eye redness with varying degrees of swelling and ocular discharge, accompanied by clinical symptoms including pain, itching, and a foreign body sensation. The highly contagious infection lasts for 2-3 weeks, with patients remaining contagious for 10-14 days after the first onset of disease. Transmission to family members or close contacts is extremely common and patients are advised to avoid work and/or school until symptoms fully resolve.
About Brandon Capital and the MRCF
Brandon Capital Partners is a venture capital firm that manages the Medical Research Commercialisation Fund (MRCF), Australia and New Zealand’s largest life science investment fund, with AU$505 million under management.
The MRCF is a unique collaboration between major Australian superannuation funds, the Australian and New Zealand governments, Australian state governments and more than 50 leading medical research institutes and research hospitals. The MRCF supports the development and commercialization of early-stage biomedical discoveries originating from member research organizations, providing both capital and expertise to guide the successful development of new therapies.
For more information about Brandon Capital Partners, visit http://www.brandoncapital.com.au/.
About Okogen
Okogen is a biotechnology company focused on developing therapeutics to help patients with ocular infections. The lead candidate is OKG-0301, a potent, broad-spectrum antiviral that functions intracellularly to inhibit viral replication. This mechanism of action provides opportunity to address adenoviral infections of the eye as well as other classes of viruses that are active in the ocular space, including herpes simplex virus, varicella-zoster virus, picornavirus, and more.
For more information about Okogen, visit http://www.okogen.com.