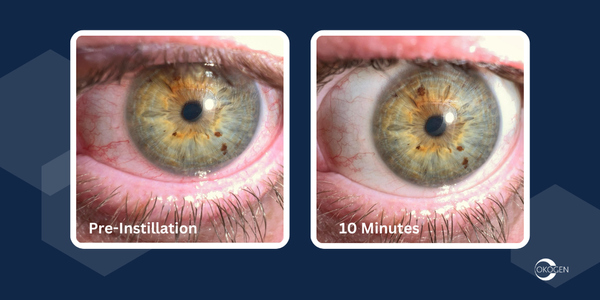
Plano, TX – December 11, 2024 – Okogen Inc., a company specializing in innovative eyecare solutions, today announced the successful completion of $3.3 million in financing. The funding will advance the company through the completion of the Phase 2b clinical program for acute infectious conjunctivitis (“AIC”) and the development of an AI-driven image based evaluation platform designed for telehealth and at home application. These initiatives reinforce Okogen’s mission to address the unmet needs of the AIC market while exploring complementary pipeline opportunities. A clinical data readout from the Phase 2b program is anticipated in Q4 2025.
“We are excited to announce the completion of our latest financing and the launch of our AI-enabled platform,” said Joshua Moriarty, CEO of Okogen. “This milestone reinforces our commitment to delivering comprehensive therapeutic solutions for patients suffering from AIC. Our focus remains on developing innovative, first-in-class products that improve outcomes for patients and efficiency for providers alike.”
The Series B funding supports pivotal transition for Okogen from a single-asset company focused solely on OKG-301, a viral conjunctivitis treatment, to a broader platform approach. This platform combines a novel AI and image based patient evaluation tool with the development of OKG-0303, a triple-combination eyedrop targeting bacterial and viral conjunctivitis. By integrating evaluation and treatment, Okogen aims to address significant gaps in managing this highly contagious condition.
“The global market for treating acute infectious conjunctivitis is vast, with millions of cases diagnosed annually across all age groups,” said Bradford Conlan, Chairman of the Board. “This condition poses substantial healthcare costs and productivity losses. Our AI-enabled platform offers a comprehensive solution to aid in patient evaluation while delivering time and cost savings for patients, payers, and healthcare providers.”
Headquarters Relocation to Plano, Texas
In line with its growth strategy, Okogen has relocated its headquarters from San Diego, California, to Plano, Texas. The move positions the company closer to critical resources and infrastructure, further accelerating the development and commercialization of its groundbreaking therapies.
About Acute Infectious Conjunctivitis
Pink eye (acute infectious conjunctivitis) is when the conjunctiva is irritated by a viral or bacterial infection. The current conjunctivitis market is USD $4.2 billion annually and is estimated to grow beyond USD $6.0 billion by 2031. Bacterial and viral conjunctivitis are easily spread from person to person if left untreated or properly diagnosed. There are currently no approved treatments for adenoviral conjunctivitis, comprising +90% of all viral ocular infections.